Clinical Research and Evidence-based Clinical Practice: from Literature Review to Meta-analysis and Clinical Practice Guidelines
Learning outcomes of the course:
This course aims to update professionals and researchers working in the areas of vision sciences or any other biomedical field in the best practices in clinical research and clinical practice guidelines. It also intends that researchers and professionals know the methodologies and statistical procedures that support the best practices in research and clinical practice.
Directed to PhD Students and final year MSc Students in any field related with clinical research and clinical practice.
At the end of the course, students should be able to:
- To know the sources of scientific knowledge and its impact in the clinical scope.
- To know the most frequent experimental designs, their advantages and disadvantages.
- To know the procedures of good practices in clinical research.
- To know the statistical procedures used in clinical research.
- Perform statistical analysis procedures on clinical research data
- Perform a systematic review or meta-analysis
- Interpret the results of a meta-analysis
- Apply scientific knowledge in clinical practice.
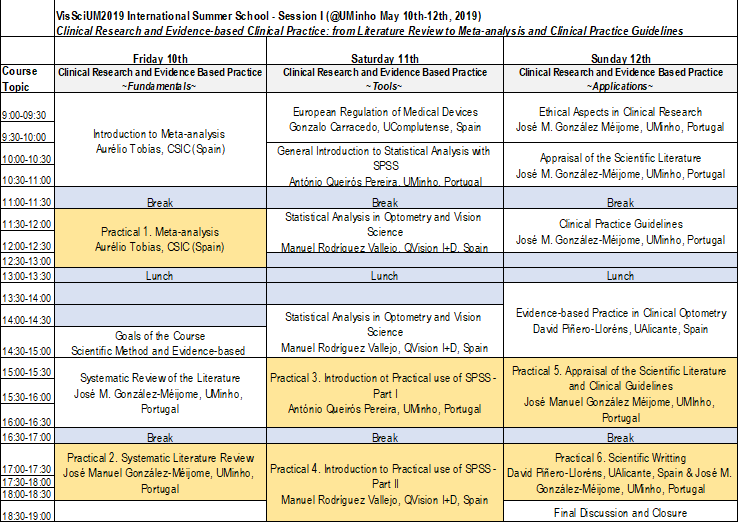
Syllabus:
Theoretical Component
- Ethical aspects in clinical research
- European regulation of medical devices
- Clinical research and scientific evidence
- New technologies applied to clinical research
- Statistical methodologies in clinical research
- Systematic reviews
- Meta-analyzes
- Evidence-based clinical practice
- Guidelines for clinical guidance in vision care
Practical and Theoretical-practical component
- Databases, systematic review and computer tools
- Research on systematic reviews and meta-analyzes
- Statistical procedures in meta-analyzes
- Statistical analysis in clinical research
- Advanced computational tools in vision science
General books and papers:
- APMCG. Investigação passo a passo. Perguntas e respostas para a investigação clínica. Núcleo de Investigação APMCG 1ª Edição. Lisboa. 2008.
- Revisiones Sistematicas en las Ciencias de la vida (Spanish Text). Jose Luis R. Martin; Aurelio Tobias Garces; Teresa Seoane Pillado. FISCAM. 2004.
- APPRAISAL OF GUIDELINES FOR RESEARCH & EVALUATION - AGREE II. Agree Trust. Available at: https://www.agreetrust.org/wp-content/uploads/2013/06/AGREE_II_Portuguese.pdf. Accessed: November 2018
- Medical device directive and Medical device regulation. European Commission. Available at: https://ec.europa.eu/growth/sectors/medical-devices/regulatory-framework_en. Accessed: November 2018
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. 2017;83:65-74.
- Zaki M, Pardo J, Carracedo G. A review of international medical device regulations: Contact lenses and lens care solutions. Contact Lens Anterior Eye. 2018. doi.org/10.1016/j.clae.2018.11.001
- Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Clinical guidelines: developing guidelines. BMJ. 1999;318(7183):593-6.
- Seto K, Matsumoto K, Kitazawa T, Fujita S, Hanaoka S, Hasegawa T. Evaluation of clinical practice guidelines using the AGREE instrument: comparison between data obtained from AGREE I and AGREE II. BMC Res Notes. 2017;10(1):716.
- Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
- Lefebvre C, Glanville J, Wieland LS, Coles B, Weightman AL. Methodological developments in searching for studies for systematic reviews: past, present and future? Syst Rev. 2013;2:78.